The condensation power process = ConPowP
Can we double the efficiency of power plants with the condensation power process?
We THINK SO!
We are a small team thinking “BIG” to make this planet a little better.
This website is about our first project that could revolutionize the way power plants generate electricity. We invite you to join us in a venture with exciting possibilities and projects.
We are open to ideas and suggestions and if you would like to contact us, you will find our email address at the bottom of the page.
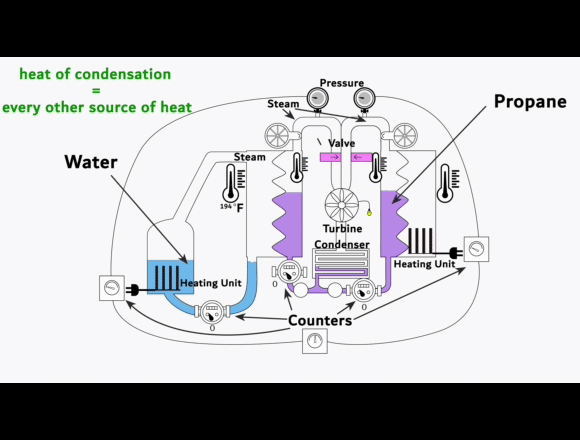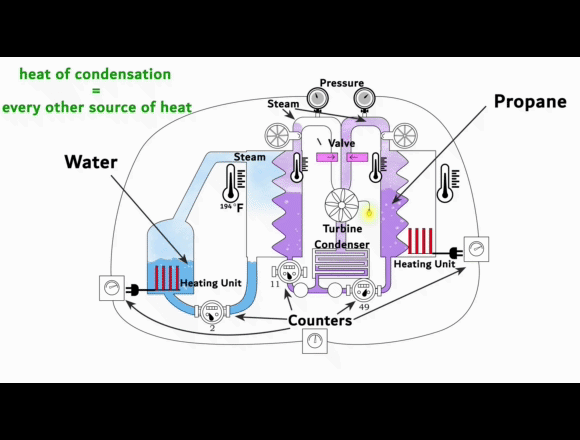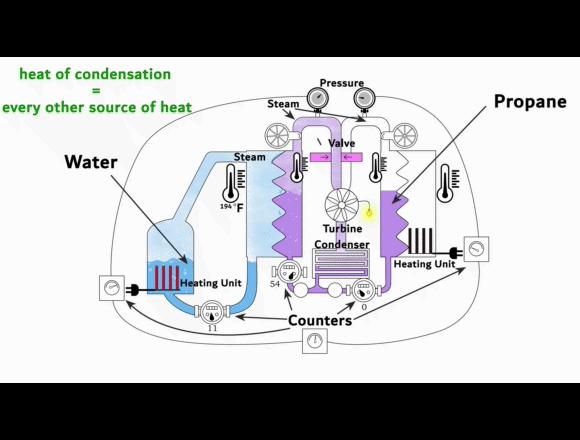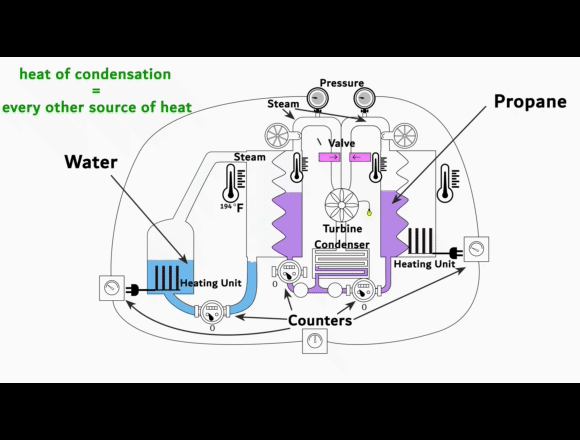
On the left side is a video-link of an experimental setup
showing the exact process. This is NOT some fancy new machine that creates energy out of nothing or from some exotic energy source. Instead, it shows that the phase change from vapor to liquid can harness organic gases to generate electricity.
The experimental setup is intended to be understood as a guide for replication.
When replicating the experiment, make sure that the heat losses through pipes etc. are the same on both sides.
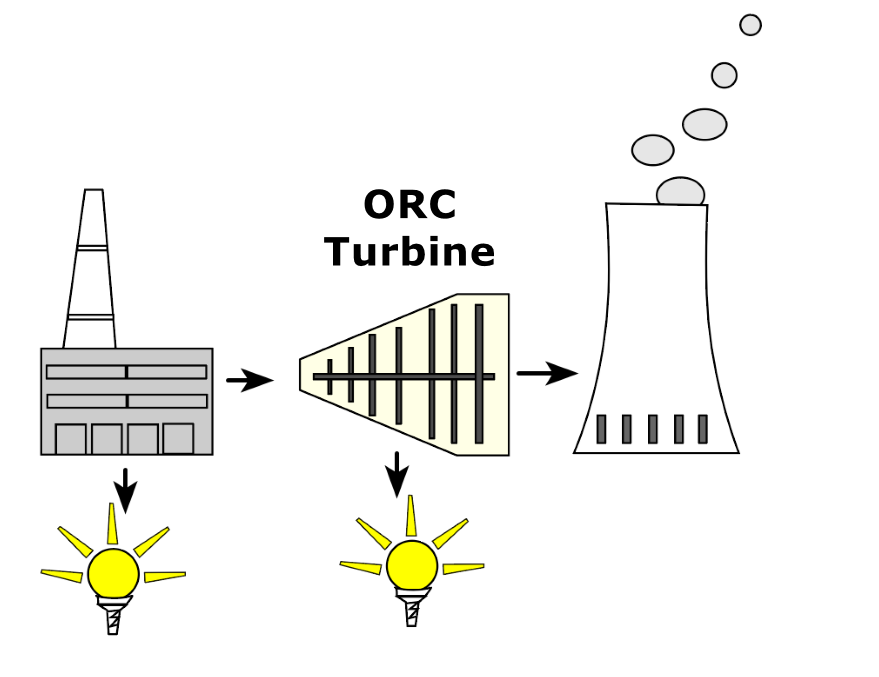
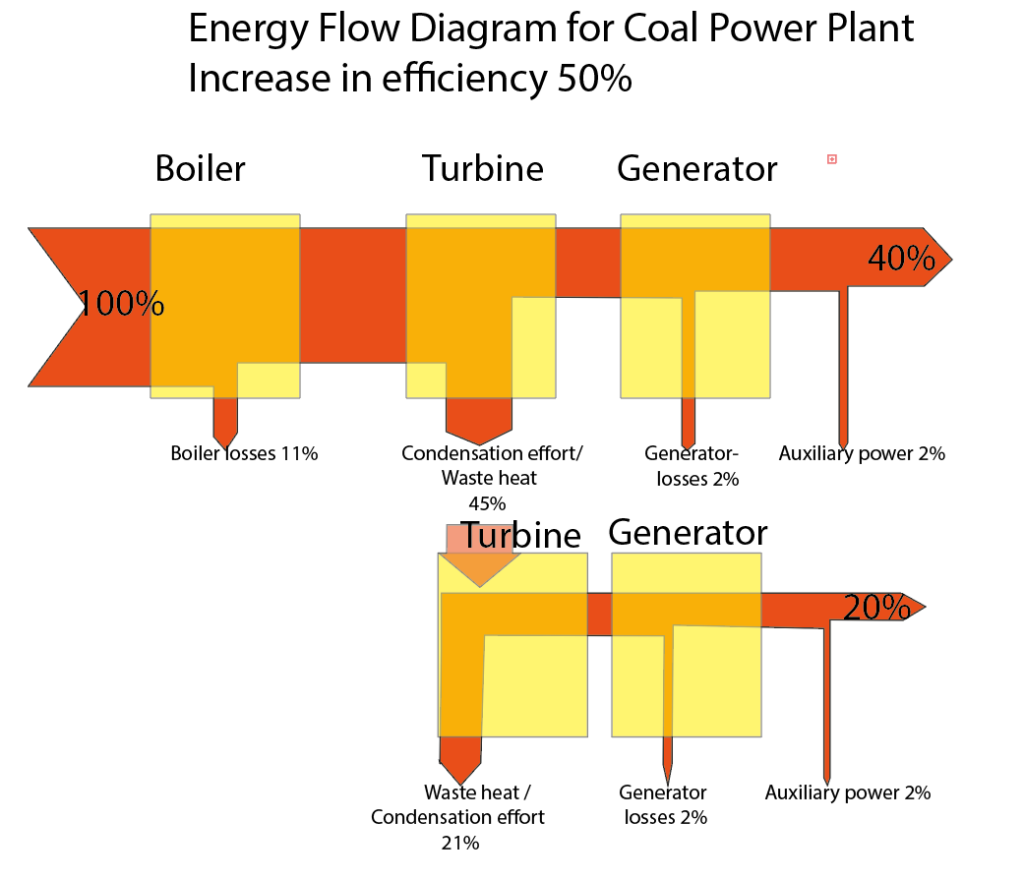
About the condensation power process
How can this invention be used to almost double the electricity output of power plants?
This is possible by heating an organic gas with the phase change (steam to liquid) of the water vapor produced by power plants and passing it through an electricity-generating ORC turbine. The condensing power process could be used in France, for example. France does not need to build any new nuclear power plants if it integrates the condensing power process into its existing power plants.
In contrast to previous ORC processes, this ORC turbine does not use the waste heat from gas-fired power plants and other industrial processes. Instead, it uses the condensation power process to generate kinetic and electrical energy.
The recipient of thermal energy cannot recognise from the heat received whether the heat was generated by burning oil, gas, waste or GEOThermie, or whether it comes from condensation.
Jasper, KonKraV
Further explanations
The heat of condensation from water vapor can be used to vaporize and superheat propane. This superheated gas can then be fed into a turbine to generate electricity. The key to this process lies in the difference between the heat of condensation of the water vapor compared to the enthalpy of vaporization of the propane. Propane requires less energy than water to turn it from liquid to gas and then it can be superheated to almost the same temperature.
When a liquid evaporates, it absorbs energy to change into the vapor phase. This energy per mass is known as the enthalpy of vaporization. Separation energy and expansion energy are required during the transition from liquid to vapor. Separation energy separates molecules so that they can move freely in the vapor, and expansion energy gives them space. The higher the heat of condensation of the water vapor and the lower the enthalpy of vaporization of the organic gas, the more thermal energy can be used to superheat the organic gas.
More superheat energy in the propane means more energy that can be converted into work output by the turbine. The conditions in the previous experimental setup already show that the organic gas can absorb enough heat energy to make its use in a turbine profitable. This energy is supplied as heat and released again during condensation. Evaporation and condensation are reversible and do not cause any losses when alternating. At thermal equilibrium, vapors contain both processes simultaneously. The amount of energy for evaporation and condensation at the same temperature is ultimately the same. As the temperature rises, both values decrease regardless of the type of gas. And above the critical point, they no longer exist.